| |
| 28/03/2012 |
|
|
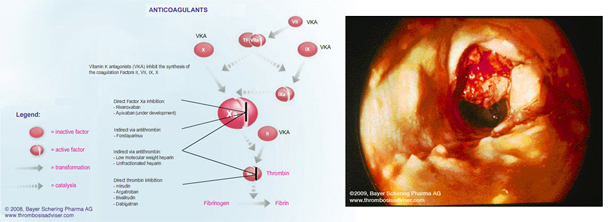
Rivaroxaban has been evaluated in a number of clinical settings,
including the prevention and treatment of venous thromboembolism and stroke prophylaxis in atrial
fibrillation. Despite medical therapy after an acute coronary syndrome, patients continue to be at risk for recurrent cardiovascular events.
Our double-blind, placebo-controlled trial, was specifically designed to randomize 15,526 patients with a recent acute coronary syndrome to receive twice-daily doses of either 2.5 mg
or 5 mg of rivaroxaban or placebo for a mean of 13 months and up to 31 months.
In our study, rivaroxaban significantly reduced the primary efficacy end point of death from cardiovascular causes, myocardial infarction, or stroke in patients with a recent acute coronary syndrome. The two doses of rivaroxaban significantly reduced the primary efficacy end point of death from cardiovascular causes, myocardial infarction, or stroke, as compared with placebo, with rates of 8.9% and 10.7%.
The two doses of rivaroxaban increased the rates of major bleeding and intracranial hemorrhage, as compared with placebo, without a significant increase in fatal bleeding. The lower dose twice-daily 2.5-mg of rivaroxaban resulted in less bleeding than the higher dose. Rivaroxaban reduced the rates of death from cardiovascular causes (2.7% vs. 4.1%, P = 0.002) and from any cause (2.9% vs. 4.5%, P = 0.002), a survival benefit that was not seen with the twice-daily 5-mg dose.
On February 28, 2012 Bayer, together with its cooperation partner, Janssen Research & Development, L.L.C., announced that the US Food and Drug Administration (FDA) has granted Priority Review designation to the supplemental New Drug Application (sNDA) filed on December 29, 2011 for the oral anticoagulant Xarelto® (rivaroxaban) in combination with standard antiplatelet therapy to reduce the risk of (thrombotic) cardiovascular events in patients with Acute Coronary Syndrome (ACS). The FDA grants Priority Review to medicines that offer advances in care or that provide a treatment where no adequate therapy exists(http://www.bayer.com/en/News-Detail.aspx?newsid=15745 )
|
Rivaroxaban in Patients with a Recent
Acute Coronary Syndrome
Jessica L. Mega, M.D., M.P.H., Eugene Braunwald, M.D., Stephen D. Wiviott, M.D., Jean-Pierre Bassand, M.D.,
Deepak L. Bhatt, M.D., M.P.H., Christoph Bode, M.D., Paul Burton, M.D., Ph.D., Marc Cohen, M.D.,
Nancy Cook-Bruns, M.D., Keith A.A. Fox, M.B., Ch.B., Shinya Goto, M.D., Sabina A. Murphy, M.P.H.,
Alexei N. Plotnikov, M.D., David Schneider, M.D., Xiang Sun, Ph.D., Freek W.A. Verheugt, M.D.,
and C. Michael Gibson, M.D., for the ATLAS ACS 2–TIMI 51 Investigators*
The New England Journal of Medicine
n engl j med 366;1 nejm.org january 5, 2012
|
|

